PHYSICS(P2) 2022 May/June Question 1 Activity
Activity Summary
0 of 10 Questions completed
Questions:
Information
You have already completed the activity before. Hence you can not start it again.
Activity is loading…
You must sign in or sign up to start the activity.
You must first complete the following:
Results
Results
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Physical Sciences Gr12 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Current
- Review / Skip
- Answered
- Correct
- Incorrect
-
Question 1 of 10
1. Question
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions.
Each question has only ONE correct answer. Choose the answer and write only the
letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK,
e.g. 1.11 E.
This is how your instructions will be in the exam. For this pratice, just select the correct answer.1.1
Which ONE of the following compounds has the LOWEST melting point?CorrectIncorrect -
Question 2 of 10
2. Question
1.2
When
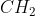 =
= 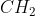 is converted to
is converted to 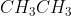 ,
,
the type of reaction is …CorrectIncorrect -
Question 3 of 10
3. Question
1.3
Which ONE of the following compounds in solution will change the colour of
bromothymol blue?CorrectIncorrectHint
Bromothymol blue is a pH indicator. It is mostly used in applications that require measuring substances that would have a relatively neutral pH. A common use is for measuring the presence of carbonic acid in a liquid. Bromothymol blue (BMB) is an indicator dye that turns yellow in the presence of acid. When carbon dioxide is added to the solution, it creates carbonic acid, lowering the pH of the solution.
-
Question 4 of 10
4. Question
1.4
Two DIFFERENT samples of IMPURE CaCO3 of EQUAL masses react with
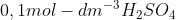 . Assume that the impurities do not react.
. Assume that the impurities do not react.
The graph below shows the volume of CO2(g) produced for each reaction.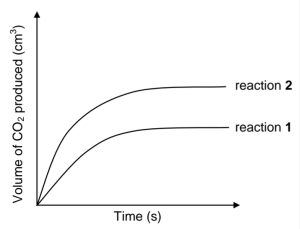
When compared to reaction 2, which ONE of the following statements BEST
explains the curve obtained for reaction 1?CorrectIncorrect -
Question 5 of 10
5. Question
1.5
 CorrectIncorrect
CorrectIncorrect -
Question 6 of 10
6. Question
1.6
 CorrectIncorrect
CorrectIncorrect -
Question 7 of 10
7. Question
1.7
Dilute nitric acid is added to distilled water at 25 °C.
How will this affect the hydronium ion concentration [H3O+] and the ionisation
constant (Kw) of water at 25 °C? CorrectIncorrect
CorrectIncorrect -
Question 8 of 10
8. Question
1.8
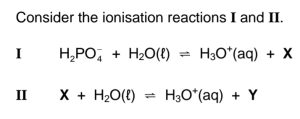
Which ONE of the following combinations represents the formulae of X and Y
respectively?
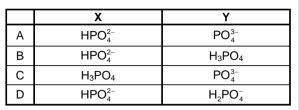 CorrectIncorrect
CorrectIncorrect -
Question 9 of 10
9. Question
1.9
 CorrectIncorrect
CorrectIncorrect -
Question 10 of 10
10. Question
1.10
The following reaction takes place in an electrochemical cell:
CuCℓ2(aq) → Cu(s) + Cℓ2(g)
Which ONE of the following is CORRECT for this cell?CorrectIncorrect

