Physics Grade12 2023 November Paper2 Exam Practice Activity
Activity Summary
0 of 10 Questions completed
Questions:
Information
You have already completed the activity before. Hence you can not start it again.
Activity is loading…
You must sign in or sign up to start the activity.
You must first complete the following:
Results
Results
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Physical Sciences Gr12 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Current
- Review / Skip
- Answered
- Correct
- Incorrect
-
Question 1 of 10
1. Question
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions.
Each question has only ONE correct answer. Choose the answer and write only the
letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK,
e.g. 1.11 E.This is how your instructions will be in the exam.For this activity, just select the correct answer.
1.1 Which ONE of the following represents a straight chain SATURATED
hydrocarbon?CorrectIncorrect -
Question 2 of 10
2. Question
1.2 Which ONE of the following is a SECONDARY alcohol?
CorrectIncorrect -
Question 3 of 10
3. Question
1.3 Which ONE of the following is a HYDROLYSIS reaction?
CorrectIncorrect -
Question 4 of 10
4. Question
1.4 Hydrochloric acid reacts with EXCESS zinc:
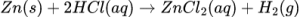
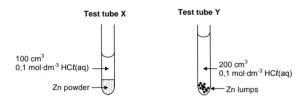
How will the INITIAL rate of reaction and FINAL VOLUME of H2(g) produced
in test tube Y compare with that in test tube X?
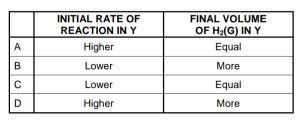 CorrectIncorrect
CorrectIncorrect -
Question 5 of 10
5. Question
1.5 The diagram below represents a mixture of NO2(g) and N2O4(g) molecules at equilibrium in a 1 dm3
container at T °C.
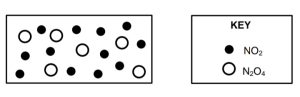
The balanced equation for this reaction is:
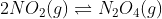
Which ONE of the following is TRUE for the value of the equilibrium constant,
Kc, for the reaction at T °C?CorrectIncorrect -
Question 6 of 10
6. Question
1.6 A reaction is at equilibrium in a closed container according to the following
balanced equation: 4CuO(s) ⇌ 2Cu2O(s) + O2(g)
The volume of the container is now increased while the temperature remains
constant. A new equilibrium is reached.
Which ONE of the following combinations is CORRECT for the new
equilibrium?
 CorrectIncorrect
CorrectIncorrect -
Question 7 of 10
7. Question
1.7 Nitric acid, HNO3(aq), and ethanoic acid, CH3COOH(aq), of equal volumes
and concentrations are compared.
Consider the following statements regarding these solutions:
(i) They have different pH values.
(ii) Both have the same electrical conductivity.
(iii) Both solutions require the same number of moles of KOH(aq) for
complete neutralisation.
Which of the above statement(s) is/are TRUE?CorrectIncorrect -
Question 8 of 10
8. Question
1.8 The apparatus in the diagram below is used for the titration between HCℓ(aq) and KOH(aq).
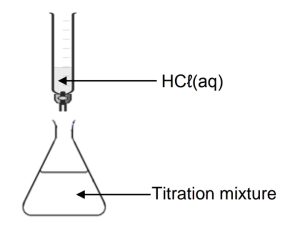
In a titration, the learner accidentally exceeds the endpoint. Which ONE of the
following will be TRUE for the titration mixture?CorrectIncorrect -
Question 9 of 10
9. Question
1.9 The following hypothetical standard reduction potentials relate to a galvanic
cell:

Consider the following statements for this galvanic cell:
(i) The emf of the cell is 0,20 V under standard conditions.
(ii) Electrode Y is the anode.
(iii) X is oxidised.
Which of the above statement(s) is/are TRUE for this galvanic cell?CorrectIncorrect -
Question 10 of 10
10. Question
1.10 Which ONE of the half-reactions below will be the MAIN reaction at the
ANODE during the electrolysis of CONCENTRATED CuCℓ2(aq)?CorrectIncorrect

