PHYSICS (P2) 2022 November Exam Practice Activity
Activity Summary
0 of 10 Questions completed
Questions:
Information
You have already completed the activity before. Hence you can not start it again.
Activity is loading…
You must sign in or sign up to start the activity.
You must first complete the following:
Results
Results
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Physical Sciences Gr12 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Current
- Review / Skip
- Answered
- Correct
- Incorrect
-
Question 1 of 10
1. Question
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions.
Each question has only ONE correct answer. Choose the answer and write only the
letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK,
e.g. 1.11 E.
This is how your instractions for the exam will be. Fir this practice, just select the correct answer1.1
Which ONE of the following terms describes hydrocarbons that contain only
single bonds?CorrectIncorrect -
Question 2 of 10
2. Question
1.2
Which ONE of the following combinations correctly indicates the
STRONGEST intermolecular forces found in ethanoic acid and methyl
propanoate respectively?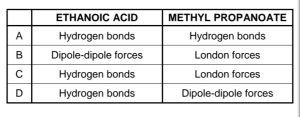 CorrectIncorrect
CorrectIncorrect -
Question 3 of 10
3. Question
1.3
A test tube contains a liquid hydrocarbon.
When bromine water (Br2) is added to the test tube, the mixture decolourises
IMMEDIATELY.
Which ONE of the following combinations correctly identifies the COMPOUND
and the TYPE OF REACTION that takes place in the test tube?
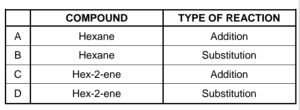 CorrectIncorrect
CorrectIncorrect -
Question 4 of 10
4. Question
1.4
Which ONE of the following statements is the CORRECT definition for the rate
of a reaction?CorrectIncorrect -
Question 5 of 10
5. Question
1.5
Consider the balanced equation for the reaction between magnesium powder
and EXCESS dilute hydrochloric acid, HCℓ(aq):
Mg(s) + 2HCℓ(aq) → MgCℓ2(aq) + H2(g)Which ONE of the following will NOT increase the rate of this reaction?
CorrectIncorrect -
Question 6 of 10
6. Question
1.6
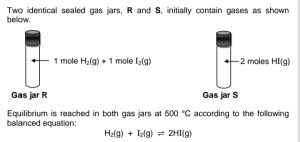
Which ONE of the following statements is TRUE at equilibrium?
CorrectIncorrect -
Question 7 of 10
7. Question
1.7
Which ONE of the following salts, when dissolved in water, will NOT change
the pH of the water?CorrectIncorrect -
Question 8 of 10
8. Question
1.8
A dilute acid is titrated against a potassium hydroxide solution, KOH(aq).
At the equivalence point the pH is 7.
Which ONE of the following combinations correctly identifies the acid and the
MOST SUITABLE indicator for this titration?
 CorrectIncorrect
CorrectIncorrect -
Question 9 of 10
9. Question
1.9
Which ONE of the following statements is TRUE for an oxidising agent?CorrectIncorrect -
Question 10 of 10
10. Question
1.10
Which ONE of the following metals will reduce Cd2+(aq) to Cd(s), but will NOT
reduce Mn2+(aq) to Mn(s)?CorrectIncorrect

